Critical point phase diagram definition
Home » Wallpapers » Critical point phase diagram definitionYour Critical point phase diagram definition images are available. Critical point phase diagram definition are a topic that is being searched for and liked by netizens today. You can Find and Download the Critical point phase diagram definition files here. Download all free vectors.
If you’re searching for critical point phase diagram definition pictures information linked to the critical point phase diagram definition interest, you have come to the ideal site. Our site frequently provides you with suggestions for downloading the maximum quality video and image content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
Critical Point Phase Diagram Definition. Critical Point Definition In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. The critical point is the end point of a phase equilibrium curve defined by a. Beyond the critical point there is no more a difference in between the liquid and vapor phase and both merge in a same phase supercritical. Another name for critical state.
 Supercritical Fluids Introduction To Chemistry From courses.lumenlearning.com
Supercritical Fluids Introduction To Chemistry From courses.lumenlearning.com
The temperature and pressure corresponding to this are known as the critical temperature and critical pressure. There are three important curves on a phase diagram which are known as fusion line sublimation line. In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. Above the critical point a substance is a supercritical. The critical point is actually the end point of the liquid vapor equilibrium curve. Critical point synonyms critical point pronunciation critical point translation English dictionary definition of critical point.
You will have noticed that this liquid-vapor equilibrium curve has a top limit labeled as C in the phase diagram in Figure 1 which is known as the critical point.
For example in the phase diagram of water the critical point is the end point of the curve that separates the liquid and vapor phases. Beyond the critical point the substance becomes. Tricritical point and phase diagram based on critical scaling in the monoaxial chiral helimagnet Cr13NbS2. In general the critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. These lines on the phase envelope are known as the bubble point and the dew point lines. The critical point is actually the end point of the liquid vapor equilibrium curve.
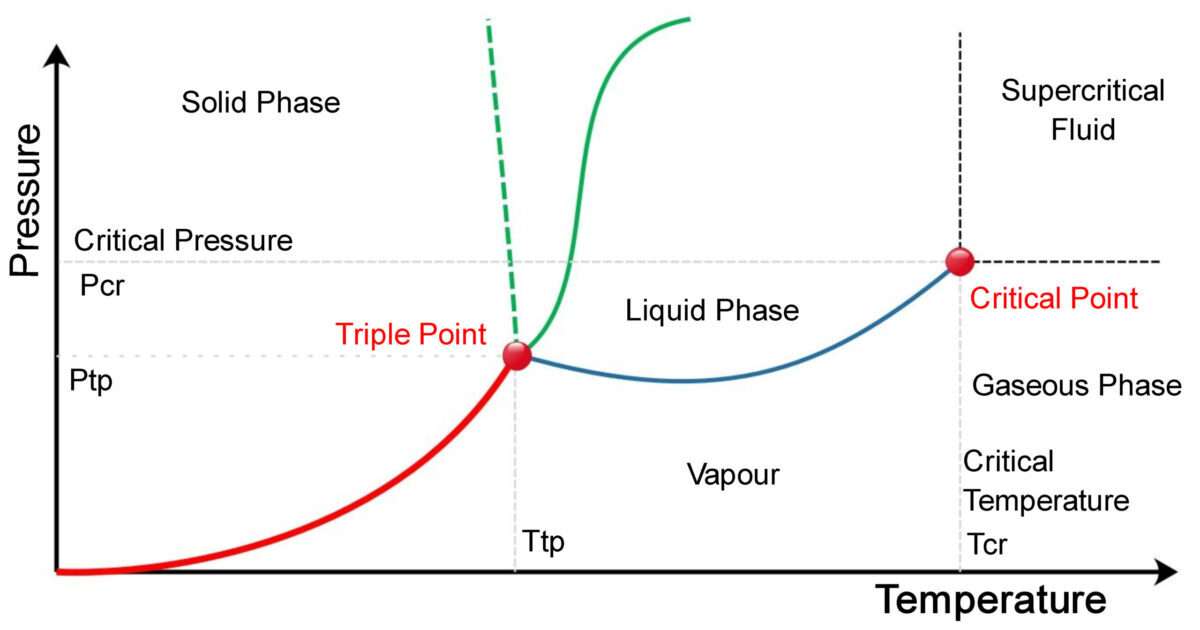 Source: chemicalengineeringworld.com
Source: chemicalengineeringworld.com
Critical point in physics the set of conditions under which a liquid and its vapour become identical see phase diagram. The critical point on the phase diagram shows where the gas and liquid states of a liquid are identical and the substance is in one phase. Above the critical point a substance is a supercritical. The temperature and pressure corresponding to this are known as the critical temperature and critical pressure. Phases a phase envelope is a region on a phase diagram that is bounded by a line which encompasses where two phases can coexist.
 Source: pediaa.com
Source: pediaa.com
The point on a phase diagram that represents the critical state of a substance. By definition it is the end point of a curve where two different phases of a given substance exist together. The point on a phase diagram that represents the critical state of a substance Meaning pronunciation translations and examples. At the liquid-gas critical point of a pure substance the distinction between liquid and gas vanishes and the. Critical point definition.
 Source: pinterest.com
Source: pinterest.com
A phase diagram is a chart showing the thermodynamic conditions of a substance at different pressures and temperatures. In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. The phase diagram of water is a pressure-temperature diagram for water that shows how all three phases solid liquid and vapor may coexist together in thermal equilibrium. The critical point is the end point of a phase equilibrium curve defined by a critical pressure Tp and critical temperature Pc. A phase diagram is simply a graph of pressure versus temperature which represents the behavior of a given substance under different combinations of pressure and temperature.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Beyond the critical point there is no more a difference in between the liquid and vapor phase and both merge in a same phase supercritical. Beyond the critical point there is no more a difference in between the liquid and vapor phase and both merge in a same phase supercritical. The critical point is the end point of a phase equilibrium curve defined by a critical pressure Tp and critical temperature Pc. In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. In general the critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase.
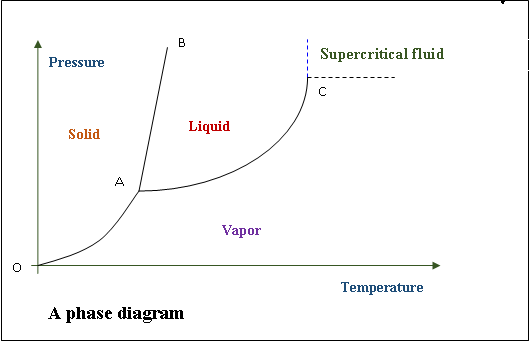 Source: pediaa.com
Source: pediaa.com
In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. The point on a phase diagram that represents the critical state of a substance Meaning pronunciation translations and examples. Critical point definition. Also called critical state. At the critical point the corresponding pressure and temperature are called the critical pressure p c and critical temperature T c of the mixture.
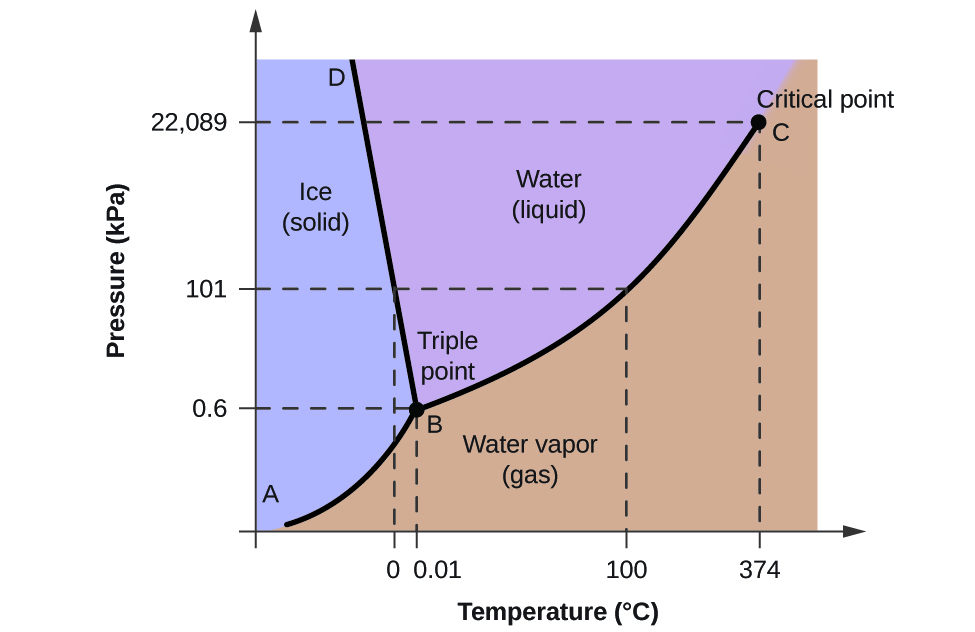 Source: opentextbc.ca
Source: opentextbc.ca
Critical point thermodynamics near the critical point all these properties change into the exact opposite water be es pressible expandable a poor lectric a bad solvent for electrolytes and prefers to mix with nonpolar gases and organic molecules at the critical point only one phase exists phase diagrams critical point triple point and phase learn. These lines on the phase envelope are known as the bubble point and the dew point lines. The point on a phase diagram that represents the critical state of a substance Meaning pronunciation translations and examples. Phase Diagram Definition. At the liquid-gas critical point of a pure substance the distinction between liquid and gas vanishes and the.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Beyond the critical point the substance becomes. What is the critical point. At the liquid-gas critical point of a pure substance the distinction between liquid and gas vanishes and the. Triple Point occurs when both the temperature and pressure of the three phases of the substance coexist in equilibrium. The critical point is actually the end point of the liquid vapor equilibrium curve.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
You will have noticed that this liquid-vapor equilibrium curve has a top limit labeled as C in the phase diagram in Figure 1 which is known as the critical point. What is the Critical Point On A Phase Diagram. At the liquid-gas critical point of a pure substance the distinction between liquid and gas vanishes and the. Phase Diagram Definition. Critical point thermodynamics near the critical point all these properties change into the exact opposite water be es pressible expandable a poor lectric a bad solvent for electrolytes and prefers to mix with nonpolar gases and organic molecules at the critical point only one phase exists phase diagrams critical point triple point and phase learn.
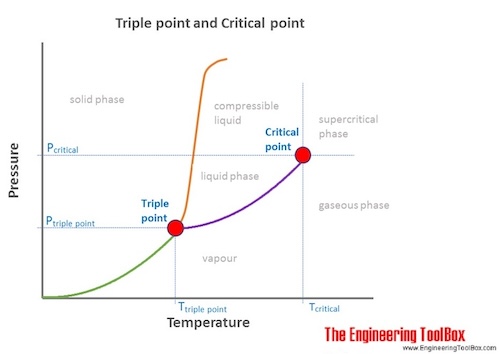 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
This is best understood by. A phase diagram is simply a graph of pressure versus temperature which represents the behavior of a given substance under different combinations of pressure and temperature. The critical point on the phase diagram shows where the gas and liquid states of a liquid are identical and the substance is in one phase. The point on a phase diagram that represents the critical state of a substance. Definition of the critical point.
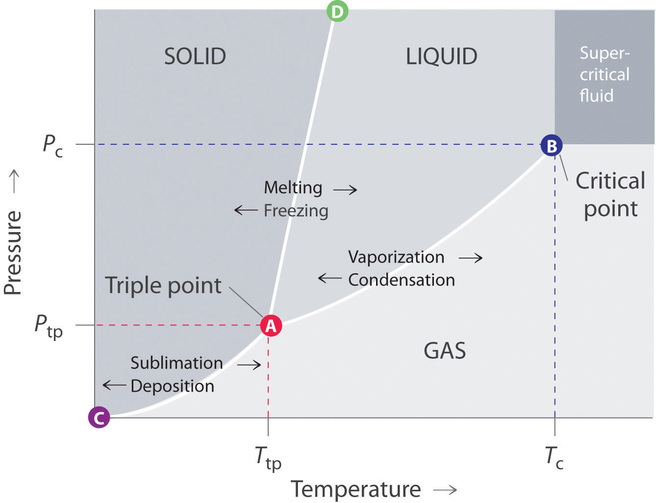 Source: chem.libretexts.org
Source: chem.libretexts.org
This is best understood by. Critical Point the point in temperature and pressure on a phase diagram where the liquid and gaseous phases of a substance merge together into a single phase. Critical pointThe critical point for a multicomponent mixture is referred to as the state of pressure and temperature at which all intensive properties of the gas and liquid phases are equal point C at Pressure-Temperature Diagram. These two lines are joined together at a point known as the critical point. Another name for critical state.
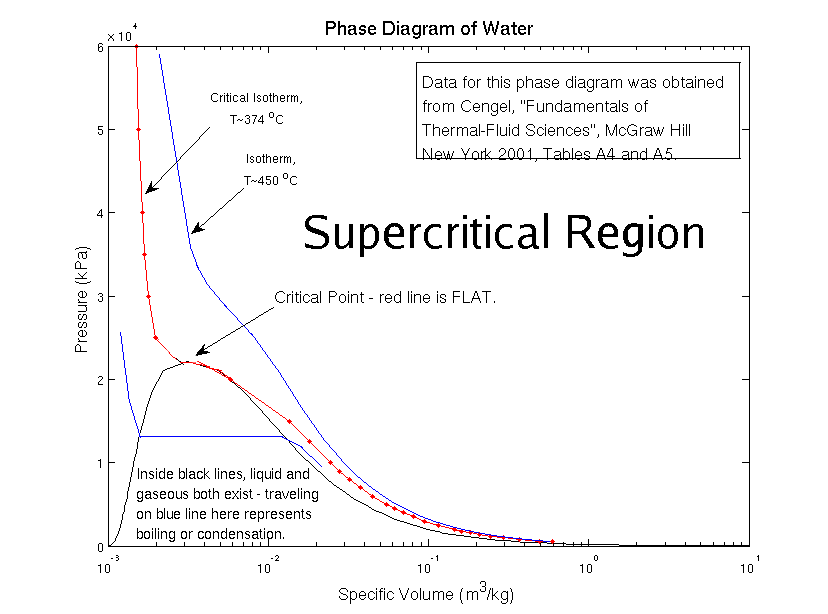 Source: web.mit.edu
Source: web.mit.edu
For each substance the conditions defining the critical point are the critical temperature the critical pressure and the critical density. Critical point thermodynamics near the critical point all these properties change into the exact opposite water be es pressible expandable a poor lectric a bad solvent for electrolytes and prefers to mix with nonpolar gases and organic molecules at the critical point only one phase exists phase diagrams critical point triple point and phase learn. Definition of the critical point. A phase diagram is simply a graph of pressure versus temperature which represents the behavior of a given substance under different combinations of pressure and temperature. These two lines are joined together at a point known as the critical point.
 Source: soft-matter.seas.harvard.edu
Source: soft-matter.seas.harvard.edu
Tricritical point and phase diagram based on critical scaling in the monoaxial chiral helimagnet Cr13NbS2. In thermodynamics a critical point or critical state is the end point of a phase equilibrium curve. A phase diagram is simply a graph of pressure versus temperature which represents the behavior of a given substance under different combinations of pressure and temperature. Beyond the critical point there is no more a difference in between the liquid and vapor phase and both merge in a same phase supercritical. Phases a phase envelope is a region on a phase diagram that is bounded by a line which encompasses where two phases can coexist.
 Source: in.pinterest.com
Source: in.pinterest.com
For example in the phase diagram of water the critical point is the end point of the curve that separates the liquid and vapor phases. Critical pointThe critical point for a multicomponent mixture is referred to as the state of pressure and temperature at which all intensive properties of the gas and liquid phases are equal point C at Pressure-Temperature Diagram. These two lines are joined together at a point known as the critical point. This is best understood by. You will have noticed that this liquid-vapor equilibrium curve has a top limit labeled as C in the phase diagram in Figure 1 which is known as the critical point.
 Source: pediaa.com
Source: pediaa.com
The critical point is actually the end point of the liquid vapor equilibrium curve. In order to understand what is meant by the critical point exactly one should have a clear idea about phase diagrams. The critical point is actually the end point of the liquid vapor equilibrium curve. Critical Point Definition In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. Critical point thermodynamics near the critical point all these properties change into the exact opposite water be es pressible expandable a poor lectric a bad solvent for electrolytes and prefers to mix with nonpolar gases and organic molecules at the critical point only one phase exists phase diagrams critical point triple point and phase learn.
 Source: researchgate.net
Source: researchgate.net
In general the critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. Critical pointThe critical point for a multicomponent mixture is referred to as the state of pressure and temperature at which all intensive properties of the gas and liquid phases are equal point C at Pressure-Temperature Diagram. At the liquid-gas critical point of a pure substance the distinction between liquid and gas vanishes and the. In order to understand what is meant by the critical point exactly one should have a clear idea about phase diagrams. Critical point thermodynamics near the critical point all these properties change into the exact opposite water be es pressible expandable a poor lectric a bad solvent for electrolytes and prefers to mix with nonpolar gases and organic molecules at the critical point only one phase exists phase diagrams critical point triple point and phase learn.
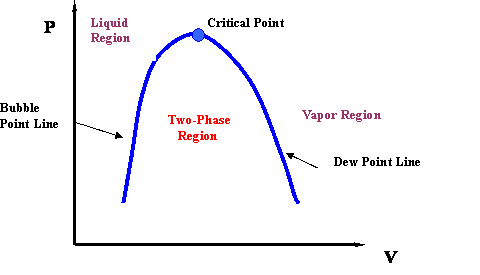 Source: e-education.psu.edu
Source: e-education.psu.edu
There are three important curves on a phase diagram which are known as fusion line sublimation line. The critical point is the end point of a phase equilibrium curve defined by a critical pressure Tp and critical temperature Pc. The temperature and pressure corresponding to this are known as the critical temperature and critical pressure. Critical point definition. Tricritical point and phase diagram based on critical scaling in the monoaxial chiral helimagnet Cr13NbS2.
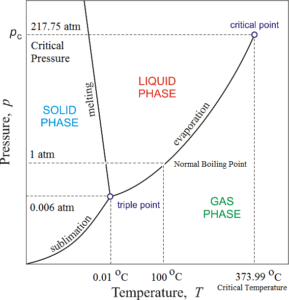 Source: thefactfactor.com
Source: thefactfactor.com
Tricritical point and phase diagram based on critical scaling in the monoaxial chiral helimagnet Cr13NbS2. Critical point definition. By definition it is the end point of a curve where two different phases of a given substance exist together. The critical point on the phase diagram shows where the gas and liquid states of a liquid are identical and the substance is in one phase. In thermodynamics a critical point or critical state is the end point of a phase equilibrium curve.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The temperature and pressure corresponding to this are known as the critical temperature and critical pressure. You will have noticed that this liquid-vapor equilibrium curve has a top limit labeled as C in the phase diagram in Figure 1 which is known as the critical point. Beyond the critical point the substance becomes. In a phase diagram The critical point or critical state is the point at which two phases of a substance initially become indistinguishable from one another. The regions around the lines show the phase of the substance and the lines show where the phases are in equilibrium.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title critical point phase diagram definition by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.