D orbital splitting diagram
Home » Wallpapers » D orbital splitting diagramYour D orbital splitting diagram images are available. D orbital splitting diagram are a topic that is being searched for and liked by netizens now. You can Find and Download the D orbital splitting diagram files here. Get all free photos.
If you’re looking for d orbital splitting diagram pictures information related to the d orbital splitting diagram keyword, you have pay a visit to the ideal blog. Our site always gives you hints for refferencing the maximum quality video and picture content, please kindly surf and find more enlightening video content and graphics that fit your interests.
D Orbital Splitting Diagram. Square Planar D Orbital Splitting Diagram 02092018 02092018 6 Comments on Square Planar D Orbital Splitting Diagram Crystal Field Theory CFT is a model that describes the breaking of degeneracies of electron In a tetrahedral crystal field splitting the d-orbitals again split into two groups with an energy difference of Δtet. Calculate the CFSE for each complex in terms of the octahedral splitting energy and the pairing energy. Placing a charge of 1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies. This electron could be used to pair one of the electrons in the lower energy t 2 g set of orbitals or it could be placed in one of the higher energy e g orbitals.
 A Splitting Of The D Orbital For A Cr 3 Cation In An Octahedral Download Scientific Diagram From researchgate.net
A Splitting Of The D Orbital For A Cr 3 Cation In An Octahedral Download Scientific Diagram From researchgate.net
A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals. Placing a charge of 1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies. Square pyramidal d z2x2-y d xy d yzxz 5. In this case the splitting energy is less than the pairing energy so the 4th and 5th electrons go into the e g orbitals. The d orbital splitting diagram for a tetrahedral coordination environment is shown below. The d orbitals fill with 8 electrons then with a low spin configuration.
A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals.
D orbital splitting diagrams In the limit of going from ML 6 to ML 4. Square Planar D Orbital Splitting Diagram 02092018 02092018 6 Comments on Square Planar D Orbital Splitting Diagram Crystal Field Theory CFT is a model that describes the breaking of degeneracies of electron In a tetrahedral crystal field splitting the d-orbitals again split into two groups with an energy difference of Δtet. In the picture the metal atom is at the centre of the cube and the circle represent the ligands. Given this diagram and the axes in the accompanying picture identify which d orbitals are found at which level. The 4th and 5th electrons must choose whether to pair up with electrons already in t 2g which costs energy or to go into higher energy e g orbitals which also costs energy. This electron could be used to pair one of the electrons in the lower energy t 2 g set of orbitals or it could be placed in one of the higher energy e g orbitals.
 Source: researchgate.net
Source: researchgate.net
In the picture the metal atom is at the centre of the cube and the circle represent the ligands. The energy separation between them is called the crystal field splitting parameter. The crystal field splitting in the tetrahedral field is intrinsically smaller than in the octahedral fieldfieldForFor mostmost purposespurposes thethe relationshiprelationship maymay bebe representedrepresented asas Δ t 49 Δo. Square pyramidal d z2x2-y d xy d yzxz 5. The d x 2 y 2 and d z 2 orbitals increase in energy while the d xy d xz and d yz orbitals decrease in energy.
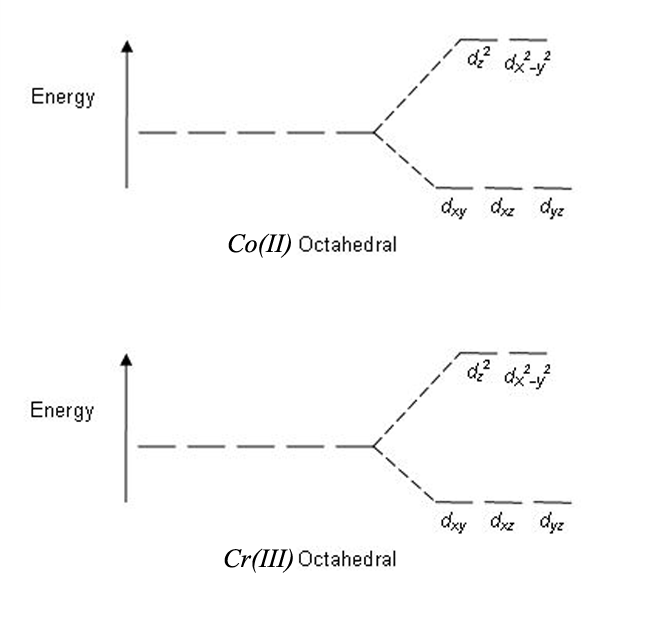 Source: chegg.com
Source: chegg.com
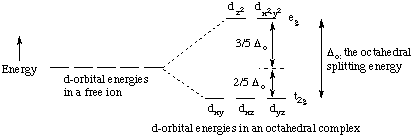
The degenerate d-orbitals in a spherical field environment split into two levels ie e g and t 2g in the presence of ligands. Concise summary of d-orbital splitting diagrams for square planar transition metal complexes which we propose may be used as an updated reference in chemical education. A low-spin case for example. The interaction between its d-electrons and the non-bonding electrons of the ligands due to orbital shape. The degenerate d-orbitals in a spherical field environment split into two levels ie e g and t 2g in the presence of ligands.
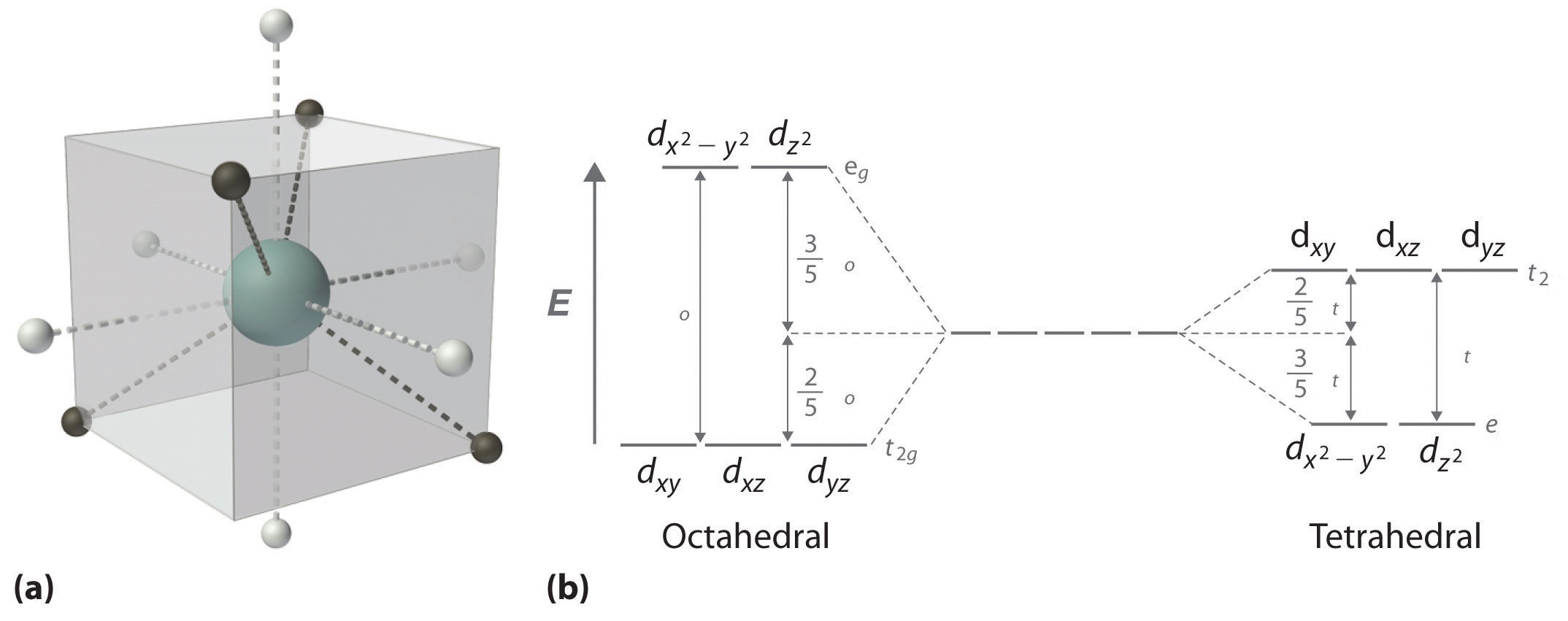 Source: chem.libretexts.org
Source: chem.libretexts.org
A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals. In this case the splitting energy is less than the pairing energy so the 4th and 5th electrons go into the e g orbitals. D-orbital diagram for FeH 2 O 6 3. D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns. Octahedral to square planar 2 e g t 2g d xz d yz d xy d z2 d x-y2.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
D orbital splitting diagrams In the limit of going from ML 6 to ML 4. The 6 ligands are put on the x y z axes black dots below Two d-orbitals are pointing right at the ligands anti-bonding. The d orbital splitting diagram for a tetrahedral coordination environment is shown below. We can use the relative energy levels of the d orbitals in a given complex to calculate whether the overall energy would be higher or lower in a high-spin vs. The average energy of the five d orbitals is the same as for a spherical distribution of a 6 charge however.
 Source: id.wikipedia.org
Source: id.wikipedia.org
Three d-orbitals are pointing in-between ligands nonbonding. Ie an octahedral Ligand Field. You can see that an even number of d orbitals will get filled dyzdxzdz2dxy with an even number of 3d electrons. A low-spin case for example. A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals.
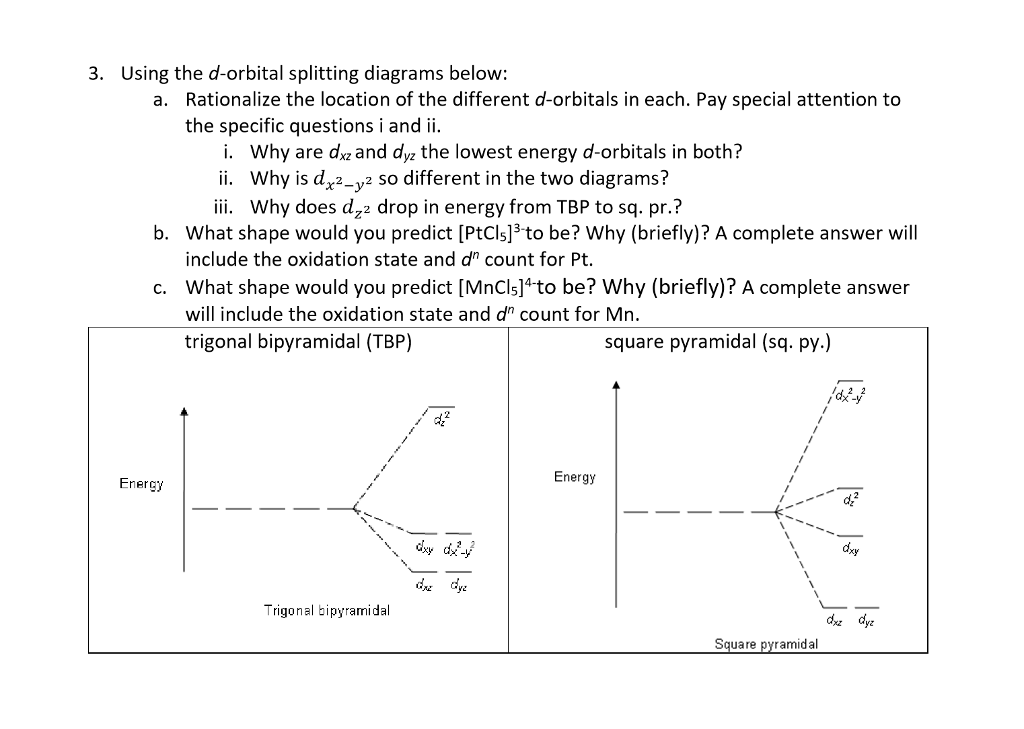 Source: chegg.com
Source: chegg.com
CRYSTAL FIELD SPLITTING DIAGRAMS. The degenerate d-orbitals in a spherical field environment split into two levels ie e g and t 2g in the presence of ligands. This electron could be used to pair one of the electrons in the lower energy t 2 g set of orbitals or it could be placed in one of the higher energy e g orbitals. A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals. D Orbitals in a Octahedral Ligand Field Lets consider d-orbitals in an octahedral complex.
 Source: researchgate.net
Source: researchgate.net
D-orbital diagram see board notes A good aid to help you think about the model is the d-orbital diagram of the complex. The 6 ligands are put on the x y z axes black dots below Two d-orbitals are pointing right at the ligands anti-bonding. Follow me on Unacademy. D-orbital diagram for FeH 2 O 6 3. The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting while the energy difference between the two levels eg and t2g is called the crystal-field splitting energy.
 Source: researchgate.net
Source: researchgate.net
Applying this will transform either of these two orbitals into something that is in-between a pure mathrmd_xy and a pure mathrmd_x2-y2 orbital. Lets look at the diagram for CoNH 3 6Cl 3. The d x2 y2 and d z2 all point directly along the x y and z axes. All of the d orbitals have four lobes of electron density except for the d z2 orbital which has two opposing lobes and a doughnut of electron density around the middle. The energy separation between them is called the crystal field splitting parameter.
 Source: researchgate.net
Source: researchgate.net
A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals. When we try to add a fourth electron we are faced with a problem. The d orbitals fill with 8 electrons then with a low spin configuration. Calculate the CFSE for each complex in terms of the octahedral splitting energy and the pairing energy. Placing a charge of 1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The 4th and 5th electrons must choose whether to pair up with electrons already in t 2g which costs energy or to go into higher energy e g orbitals which also costs energy. Three d-orbitals are pointing in-between ligands nonbonding. D-orbital diagram see board notes A good aid to help you think about the model is the d-orbital diagram of the complex. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d xz d z2 d x2-y d xy d yz d xz z x y. CRYSTAL FIELD SPLITTING DIAGRAMS.
 Source: researchgate.net
Source: researchgate.net
This electron could be used to pair one of the electrons in the lower energy t 2 g set of orbitals or it could be placed in one of the higher energy e g orbitals. Octahedral transition-metal ions with d 1 d 2 or d 3 configurations can therefore be described by the following diagrams. This electron could be used to pair one of the electrons in the lower energy t 2 g set of orbitals or it could be placed in one of the higher energy e g orbitals. The energy separation between them is called the crystal field splitting parameter. The d orbitals fill with 8 electrons then with a low spin configuration.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The d x 2 y 2 and d z 2 orbitals increase in energy while the d xy d xz and d yz orbitals decrease in energy. There is a variation on how to think about d orbital splitting diagrams that can be useful in deciding how the d electrons are configured in transition metal complexes. You can see that an even number of d orbitals will get filled dyzdxzdz2dxy with an even number of 3d electrons. D-orbital diagram see board notes A good aid to help you think about the model is the d-orbital diagram of the complex. Ie an octahedral Ligand Field.
 Source: sites.google.com
Source: sites.google.com
The first three electrons go into t 2g orbitals unpaired. The degenerate d-orbitals in a spherical field environment split into two levels ie e g and t 2g in the presence of ligands. The first three electrons go into t 2g orbitals unpaired. A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals. Placing a charge of 1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies.
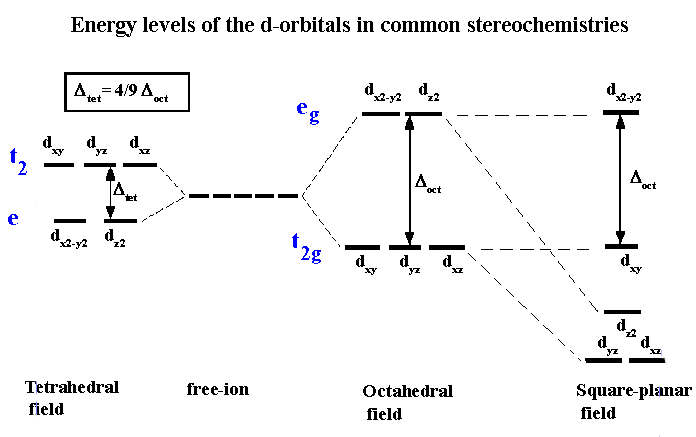 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
This electron could be used to pair one of the electrons in the lower energy t 2 g set of orbitals or it could be placed in one of the higher energy e g orbitals. The d orbitals fill with 8 electrons then with a low spin configuration. Concise summary of d-orbital splitting diagrams for square planar transition metal complexes which we propose may be used as an updated reference in chemical education. For each complex draw the d-orbital splitting diagram and show the locations of the electrons. The d-orbital splitting diagram is the inverse of that for an octahedral complex.
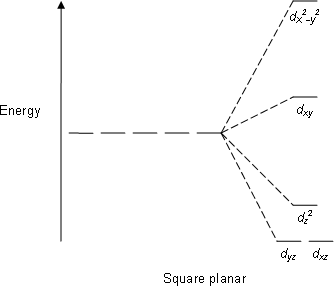 Source: socratic.org
Source: socratic.org
Applying this will transform either of these two orbitals into something that is in-between a pure mathrmd_xy and a pure mathrmd_x2-y2 orbital. A FeH 2 O 4 OH 2 magnetic moment 592 BM. Their blank d -splitting diagrams within the realm of crystal field theory are. Applying this will transform either of these two orbitals into something that is in-between a pure mathrmd_xy and a pure mathrmd_x2-y2 orbital. The first three electrons go into t 2g orbitals unpaired.
 Source: researchgate.net
Source: researchgate.net
The 4th and 5th electrons must choose whether to pair up with electrons already in t 2g which costs energy or to go into higher energy e g orbitals which also costs energy. D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns. Square pyramidal d z2x2-y d xy d yzxz 5. The d-orbital splitting diagram is the inverse of that for an octahedral complex. The d x 2 y 2 and d z 2 orbitals increase in energy while the d xy d xz and d yz orbitals decrease in energy.
 Source: youtube.com
Source: youtube.com
A low-spin case for example. A general d-orbital splitting diagram for square planar D 4h transition metal complexes can be derived from the general octahedral O h splitting diagram in which the d z 2 and the d x 2 y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy d xz and d yz orbitals. Their blank d -splitting diagrams within the realm of crystal field theory are. For each complex draw the d-orbital splitting diagram and show the locations of the electrons. Lets look at the diagram for CoNH 3 6Cl 3.
 Source: researchgate.net
Source: researchgate.net
The degenerate d-orbitals in a spherical field environment split into two levels ie e g and t 2g in the presence of ligands. Concise summary of d-orbital splitting diagrams for square planar transition metal complexes which we propose may be used as an updated reference in chemical education. For each complex draw the d-orbital splitting diagram and show the locations of the electrons. The d-orbital splitting diagram is the inverse of that for an octahedral complex. The d orbital splitting diagram for a tetrahedral coordination environment is shown below.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title d orbital splitting diagram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.