Dot diagram of sulfur
Home » Background » Dot diagram of sulfurYour Dot diagram of sulfur images are available. Dot diagram of sulfur are a topic that is being searched for and liked by netizens now. You can Get the Dot diagram of sulfur files here. Download all royalty-free images.
If you’re looking for dot diagram of sulfur images information related to the dot diagram of sulfur topic, you have pay a visit to the ideal site. Our site frequently provides you with hints for viewing the highest quality video and image content, please kindly search and locate more informative video content and graphics that match your interests.
Dot Diagram Of Sulfur. Plus sulfur is in group 6 or 16 on the periodic table so it has 6 valence electrons. Lewis dot structure will have 4 paired dots around Sulfur atom. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Electron dot diagrams are the diagrams which represent the valence electrons in an element.
 The Sulfur Cycle Sulphur Cycle Teacher Hacks Science And Technology From pinterest.com
The Sulfur Cycle Sulphur Cycle Teacher Hacks Science And Technology From pinterest.com
Decide which is the central atom in the structure. Plus sulfur is in group 6 or 16 on the periodic table so it has 6 valence electrons. The lewis dot structure for sulfur includes the upper case letter s the elements chemical symbol surrounded by two sets of paired dots and two single dots evenly spaced around the letter. Dot Diagram For So2. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Well its hard to draw in a dot cross because of the resonance but a google from looking at this diagram i can see.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom.
How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. As a result wrap around the central sulfur atoms bond pair valence electrons first see figure for step1. Sulfur having valence electrons in the 3rd energy level would also have access to the. That will normally be the least electronegative atom S. Electron dot diagram for sulfur. 2 2 2 D E F Covalent Bonding Ellesmere Ocr A Level Chemistry.
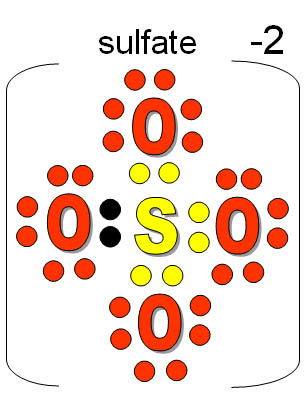 Source: pinterest.com
Source: pinterest.com
Answer 1 of 2. This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability which is easier to attain. Electron dot diagrams are the diagrams which represent the valence electrons in an element. That will normally be the least electronegative atom S. Sulfur having valence electrons in the 3rd energy level would also have access to the.
 Source: pinterest.com
Source: pinterest.com
Lewis dot diagram for so2. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Molecule shapes dot and cross diagrams bond angles for selected molecules and ions of nitrogen sulfur and chlorine using the valence-shell electron-pair repulsion model VSEPR and the dot and cross ox diagrams are presented in Lewis style The scribbles will be replaced by neat diagrams eventually. The reason for drawingcreating a lewis dot structure is that it helps one predict the kinds of bonds as well as a number of bonds that can be formed around an atom. The lewis dot structure for sulfur includes the upper case letter s the elements chemical symbol surrounded by two sets of paired dots and two single dots evenly spaced around the letter.
 Source: pinterest.com
Source: pinterest.com
The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1. Draw a trial structure by putting electron pairs around every ato. Electron dot diagram for sulfur. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Using Dot And Cross To Represent Electrons Draw A Diagram To.
 Source: pinterest.com
Source: pinterest.com
Chemical Bonding 6 Th Year Chemistry Higher Level Sinead Nolan. Elements by proton number. The Making Of Natural Iron Sulfide Nanoparticles In A Hot Vent. Here are the steps I follow when drawing a Lewis structure. That will normally be the least electronegative atom S.
 Source: pinterest.com
Source: pinterest.com
The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1. The lewis dot structure for sulfur includes the upper case letter s the elements chemical symbol surrounded by two sets of paired dots and two single dots evenly spaced around the letter. Lewis Dot Structure of SF6. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. Lewis dot of sulfur dioxide.
 Source: pinterest.com
Source: pinterest.com
Lewis dot diagram for so2. Lewis dot diagram for h2s. Sulfur having valence electrons in the 3rd energy level would also have access to the. The reason for drawingcreating a lewis dot structure is that it helps one predict the kinds of bonds as well as a number of bonds that can be formed around an atom. The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1.
 Source: pinterest.com
Source: pinterest.com
Hydrogen Hydrogen Dot Diagram. Plus sulfur is in group 6 or 16 on the periodic table so it has 6 valence electrons. The sulfur atom in the molecule gets only 8 electrons around its molecular structure. Drawing the Lewis Structure for SO 2. Draw a trial structure by putting electron pairs around every ato.
 Source: pinterest.com
Source: pinterest.com
This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability which is easier to attain. As a result wrap around the central sulfur atoms bond pair valence electrons first see figure for step1. Valence electrons in Arsenic As 5. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
The Making Of Natural Iron Sulfide Nanoparticles In A Hot Vent. The reason for drawingcreating a lewis dot structure is that it helps one predict the kinds of bonds as well as a number of bonds that can be formed around an atom. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Lewis dot diagram for so2. The electron dot diagram for a lone uncharged sulfur particle is an s with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1.
 Source: pinterest.com
Source: pinterest.com
How to draw the Lewis Structure of SO2 - with explanationCheck me out. How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. Lewis dot structure will have 4 paired dots around Sulfur atom. Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons instead of 16. Electron dot diagrams are the diagrams which represent the valence electrons in an element.
 Source: pinterest.com
Source: pinterest.com
How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. Molecule shapes dot and cross diagrams bond angles for selected molecules and ions of nitrogen sulfur and chlorine using the valence-shell electron-pair repulsion model VSEPR and the dot and cross ox diagrams are presented in Lewis style The scribbles will be replaced by neat diagrams eventually. Draw the lewis dot diagram for h2s. Electron dot diagrams are the diagrams which represent the valence electrons in an element. Sulfur has valence electrons in the 3rd energy level allowing access.
 Source: pinterest.com
Source: pinterest.com
The O 2 ion has gained two electrons in its valence shell so its Lewis electron dot diagram is as follows. Also know what is the Lewis dot structure for sulfur. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. The lewis dot structure for sulfur includes the upper case letter s the elements chemical symbol surrounded by two sets of paired dots and two single dots evenly spaced around the letter. The electron dot diagram for a lone uncharged sulfur particle is an s with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1.
 Source: pinterest.com
Source: pinterest.com
The sulfur atom in the molecule gets only 8 electrons around its molecular structure. The number of dots equals the number of valence electrons in the atom. The electrons are represented by the dots in these diagrams. The central atom here is Sulfur as it is less electronegative than Fluorine. Sulfur having valence electrons in the 3rd energy level would also have access to the.
 Source: pinterest.com
Source: pinterest.com
Well its hard to draw in a dot cross because of the resonance but a google from looking at this diagram i can see. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. H2s Dot Diagram Best Wiring Library Lewis structure of hydrogen sulfide. How to draw the Lewis Structure of SO2 - with explanationCheck me out. Electron dot diagram for sulfur.
 Source: pinterest.com
Source: pinterest.com
Lewis Dot Structure of SF6. Drawing the Lewis Structure for SO 2. That will normally be the least electronegative atom S. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
The number of dots equals the number of valence electrons in the atom. That would mean that you would have a total of eight dots around the carbon thereby filling its. The number of dots equals the number of valence electrons in the atom. The electron dot diagram for a lone uncharged sulfur particle is an s with 6 electrons arranged around it 2 orbitals with 2 electrons and 2 orbitals with 1. Draw a skeleton structure in which the other atoms are single-bonded to the central atom.
 Source: pinterest.com
Source: pinterest.com
Draw a skeleton structure in which the other atoms are single-bonded to the central atom. How to draw the Lewis Structure of SO2 - with explanationCheck me out. SBr2 Lewis structure diagram we always begin by introducing valence electrons from the central sulfur atomin step1. The O 2 ion has gained two electrons in its valence shell so its Lewis electron dot diagram is as follows. The central atom here is Sulfur as it is less electronegative than Fluorine.
 Source: pinterest.com
Source: pinterest.com
As there are 6 atoms of Fluorine there will be a formation of 6 bonds between Sulfur and Fluorine. Draw a skeleton structure in which. Lewis Dot Structure of SF6. As a result wrap around the central sulfur atoms bond pair valence electrons first see figure for step1. The lewis dot structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title dot diagram of sulfur by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.