Electron energy level diagram
Home » Background » Electron energy level diagramYour Electron energy level diagram images are available in this site. Electron energy level diagram are a topic that is being searched for and liked by netizens now. You can Get the Electron energy level diagram files here. Download all royalty-free photos.
If you’re looking for electron energy level diagram images information linked to the electron energy level diagram interest, you have visit the ideal blog. Our website frequently gives you hints for downloading the highest quality video and picture content, please kindly surf and find more informative video content and images that match your interests.
Electron Energy Level Diagram. The K shell is regarded as the nearest shell to the nucleus further followed by the L shell then the M shell and so on away from the nucleus. Quantized energy levels result from the wave behavior of particles which gives a relationship between a particles energy and its wavelengthFor a confined particle such as an electron in an atom the wave functions that have well defined energies have the form of a standing wave. An empty 2s-level of Li i exists in the energy gap and the level with one electron exists in the conduction band. E_n-frac 136 n2text eV.
 Electron Configuration Electron Configuration Teaching Chemistry Chemistry Classroom From pinterest.com
Electron Configuration Electron Configuration Teaching Chemistry Chemistry Classroom From pinterest.com
The x-axis shows the allowed energy levels of electrons in a hydrogen atom numbered from 1 to 5. The diagram below shows a photon with an energy equal to the difference between the 1st and 3rd energy levels being absorbed by an electron. For a single electron instead of per mole the formula in eV electron volts is also widely used. We know atoms have energy levels. E2 the energy needed to be at level 2. One electron volt is the.
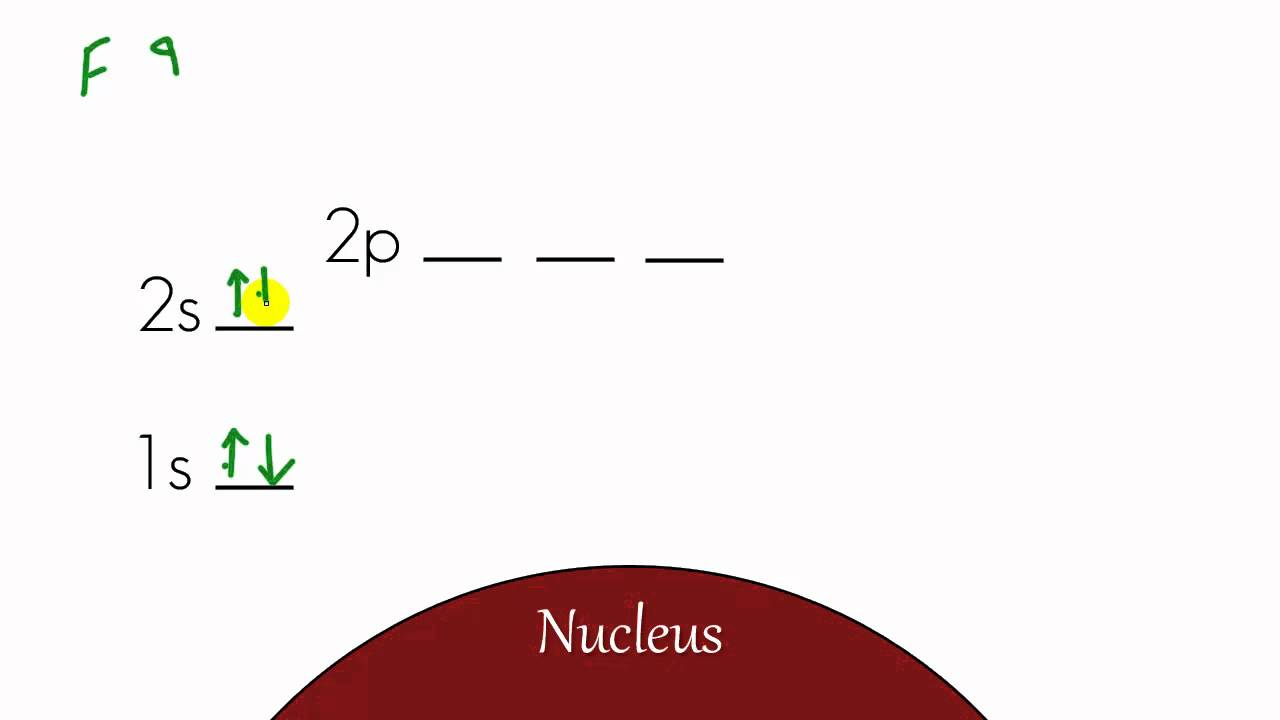
So you put 8 electrons into your energy level diagram.
The K shell is regarded as the nearest shell to the nucleus further followed by the L shell then the M shell and so on away from the nucleus. In the 4th shells they are. The latter shows strong shake-up features. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption spectrum. Therefore the electron energy levels of these five d-orbitals in mechanics forming a set five-fold degenerate energy level and the shape diagram dimensions of these orbitals identify by the principal azimuthal magnetic spin quantum number or numbers of the atom for physics or. E_n-frac 1312 n2text kJmol.
 Source: pinterest.com
Source: pinterest.com
If two electrons end up in the same orbital one arrow faces up and the other faces down. The x-axis shows the allowed energy levels of electrons in a hydrogen atom numbered from 1 to 5. The y-axis shows each levels energy in electron volts eV. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. Energy Levels Orbital Diagrams Electron Config Noble Gas - Google Slides.
 Source: pinterest.com
Source: pinterest.com
States having well-defined energies are called stationary states because they are the states that do not change. A photon is emitted or absorbed when an electron transitions from one energy state to another. E_n-frac 136 n2text eV. Energy level diagrams These diagrams show the relative energies of electrons in various orbitals under normal conditions. Lets say our pretend atom has electron energy levels of zero eV four eV six eV and seven eV.
 Source: pinterest.com
Source: pinterest.com
This is sometimes called the. When filing orbitals within any given. If two electrons end up in the same orbital one arrow faces up and the other faces down. Hf E1 - E2. The latter shows strong shake-up features.
 Source: pinterest.com
Source: pinterest.com
E n 136 n 2 eV. E1 the energy needed to be at level 1. If two electrons end up in the same orbital one arrow faces up and the other faces down. If each level has an energy value it is easy to see that the difference between the energy levels must equal the energy delivered by the photon. The Aufbau principle from the German Aufbau meaning building up construction describes a model-building method in which an atom is built up by progressively adding electrons.
 Source: pinterest.com
Source: pinterest.com
The x-axis shows the allowed energy levels of electrons in a hydrogen atom numbered from 1 to 5. We know atoms have energy levels. E_n-frac 136 n2text eV. What is an Energy Level Diagram. All the states have the same magnetic energy and that energy corresponds to the zero of magnetic energy.
 Source: pinterest.com
Source: pinterest.com
Hf E1 - E2. Electrons are always placed in the lowest energy level available 2 Hunds Rule. States having well-defined energies are called stationary states because they are the states that do not change. An empty 2s-level of Li i exists in the energy gap and the level with one electron exists in the conduction band. If each level has an energy value it is easy to see that the difference between the energy levels must equal the energy delivered by the photon.
 Source: pinterest.com
Source: pinterest.com
Thus at zero field the magnetic energy diagram for a D T and S state is quite simple. Important observations from energy level diagrams of multi electron atoms are. The diagram below shows a photon with an energy equal to the difference between the 1st and 3rd energy levels being absorbed by an electron. Electrons are always placed in the lowest energy level available 2 Hunds Rule. Therefore the electron energy levels of these five d-orbitals in mechanics forming a set five-fold degenerate energy level and the shape diagram dimensions of these orbitals identify by the principal azimuthal magnetic spin quantum number or numbers of the atom for physics or.
 Source: pinterest.com
Source: pinterest.com
E2 the energy needed to be at level 2. Thus at zero field the magnetic energy diagram for a D T and S state is quite simple. The first electron goes into the 1s orbital filling the lowest energy level first. In the second shell 2s l0 has lower energy than 2p l1 In the 3rd shell energies are in the order. Therefore the electron energy levels of these five d-orbitals in mechanics forming a set five-fold degenerate energy level and the shape diagram dimensions of these orbitals identify by the principal azimuthal magnetic spin quantum number or numbers of the atom for physics or.
 Source: pinterest.com
Source: pinterest.com
So you put 8 electrons into your energy level diagram. An electron shell or energy level can be pictured in chemistry as an orbit of electrons around an atoms nucleus. E_n-frac 1312 n2text kJmol. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Total energy of an electron in Bohrs nth stationary orbit is.
 Source: pinterest.com
Source: pinterest.com
These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption spectrum. The latter shows strong shake-up features. The Aufbau principle from the German Aufbau meaning building up construction describes a model-building method in which an atom is built up by progressively adding electrons. E n - frac2πmK2Z2e4n2h25 Or E n - frac136n26 Here TE of an e-in a stationary orbit is negative which means the electron is tightly bound to the nucleus. This is sometimes called the.
 Source: pinterest.com
Source: pinterest.com
These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption spectrum. States having well-defined energies are called stationary states because they are the states that do not change. E n - frac2πmK2Z2e4n2h25 Or E n - frac136n26 Here TE of an e-in a stationary orbit is negative which means the electron is tightly bound to the nucleus. The energy level of the electron of a hydrogen atom is given by the following formula where. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another.
 Source: pinterest.com
Source: pinterest.com
These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption spectrum. E n 136 n 2 eV. If each level has an energy value it is easy to see that the difference between the energy levels must equal the energy delivered by the photon. By default electrons are found in the lowest energy level possible close. The Aufbau principle from the German Aufbau meaning building up construction describes a model-building method in which an atom is built up by progressively adding electrons.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Therefore the electron energy levels of these five d-orbitals in mechanics forming a set five-fold degenerate energy level and the shape diagram dimensions of these orbitals identify by the principal azimuthal magnetic spin quantum number or numbers of the atom for physics or. What is energy level diagram. Figure 4 Schematic electron energy level diagram a of a core-level photoelectron ejection process one electron process b core-level photoelectron ejection process with shake-up two- electron process c schematic XPS spectrum from a plus b d Cu 2pa2 XPS spectrum for Cu in CU2O and Cu in CuO. The diagram below shows a photon with an energy equal to the difference between the 1st and 3rd energy levels being absorbed by an electron. In the second shell 2s l0 has lower energy than 2p l1 In the 3rd shell energies are in the order.
 Source: pinterest.com
Source: pinterest.com
Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. Energy Level Diagram Image will be Uploaded Soon. E_n-frac 1312 n2text kJmol. By default electrons are found in the lowest energy level possible close. An empty 2s-level of Li i exists in the energy gap and the level with one electron exists in the conduction band.
 Source: pinterest.com
Source: pinterest.com
Energy level diagrams These diagrams show the relative energies of electrons in various orbitals under normal conditions. E1 the energy needed to be at level 1. In chemistry an electron shell or energy level may be imagined as an orbit with electrons around the nucleus of an atom. Energy Level Diagram Image will be Uploaded Soon. Total energy of an electron in Bohrs nth stationary orbit is.
 Source: pinterest.com
Source: pinterest.com
2s and 2p have different energies. In Figure 14 the degenerate levels. States having well-defined energies are called stationary states because they are the states that do not change. One electron volt is the. Figure 2 shows the one-electron energy level diagram obtained with the ionic model.
 Source: pinterest.com
Source: pinterest.com
Hf E1 - E2. By default electrons are found in the lowest energy level possible close. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. We know atoms have energy levels. Lets say our pretend atom has electron energy levels of zero eV four eV six eV and seven eV.
 Source: pinterest.com
Source: pinterest.com
An empty 2s-level of Li i exists in the energy gap and the level with one electron exists in the conduction band. What is an Energy Level Diagram. The photon absorption causes the electron to transition from the 1st energy level to the 3rd. All the states have the same magnetic energy and that energy corresponds to the zero of magnetic energy. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electron energy level diagram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.