Ethylene molecular orbital diagram
Home » Background » Ethylene molecular orbital diagramYour Ethylene molecular orbital diagram images are available. Ethylene molecular orbital diagram are a topic that is being searched for and liked by netizens today. You can Find and Download the Ethylene molecular orbital diagram files here. Find and Download all free images.
If you’re searching for ethylene molecular orbital diagram pictures information connected with to the ethylene molecular orbital diagram keyword, you have pay a visit to the ideal site. Our site always gives you hints for refferencing the maximum quality video and picture content, please kindly surf and find more enlightening video content and graphics that match your interests.
Ethylene Molecular Orbital Diagram. The simplest alkene is ethene. Looking at both sigma and pi bonds. This will be done by combining two methylene. ZThe in-phase combination gave the bonding orbital.
 An Example Of A Frontier Molecular Orbital Diagram Diagram Of The Download Scientific Diagram From researchgate.net
An Example Of A Frontier Molecular Orbital Diagram Diagram Of The Download Scientific Diagram From researchgate.net
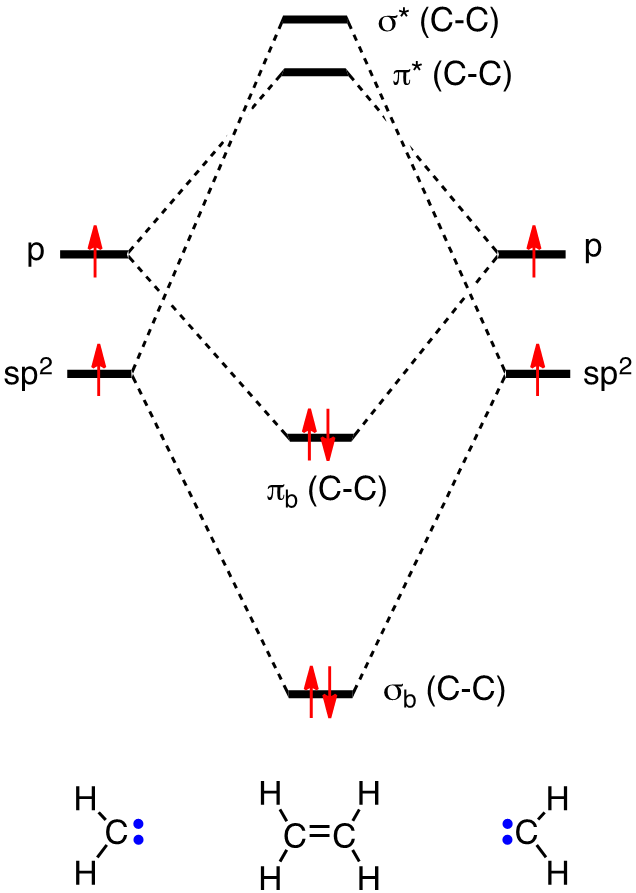
C2H4 Molecular Geometry And Bond Angles. McQuarrie Simon Physical Chemistry. In this video we will generate a qualitative MO diagram of ethene through a fragment molecular orbital approach. π Molecular Orbitals of Ethene. Ethene Ethylene Ethylene MOs. Q The overlap of the two atomic orbitals AOs results in the formation of two molecular orbitals MOs one of which is lower in energy than the original AOs the bonding MO or BMO and the other higher.
σ out is directed away from hydrogens and towards the C-C bond.
ZThe out-of-phase combination the anti-bonding orbital. ZFor ethene the σιγµα framework is created by the interaction of the sp2 hybrid orbitals of the C atoms and H1s orbitals. Make a list of all the molecular orbitals in the molecule. Only consider the first-order mixings. At this stage we note that from our N pz orbitals we will obtain N π orbitals. A Molecular Approach p.
 Source: researchgate.net
Source: researchgate.net
In this manner ethane can be constructed from MOs of two. Ethylene is the simplest molecule that has a double bond. In chapter 1 we saw that the molecular orbitals of H 2 are created by the combination of 1s orbitals. Its chemistry is dominated by two frontier orbitals that is the Highest Occupied Molecular Orbital HOMO and the Lowest Unoccupied Molecular Orbital LUMO. Means molecular orbitals σ π C from left end of double bond MO.
 Source: researchgate.net
Source: researchgate.net
In this manner ethane can be constructed from MOs of two. The out-of-phase combination the anti-bonding orbital. Arrange the molecular orbitals in order using the general order on the previous page. The diagram below shows the bond lengths and hydrogen-carbon-carbon bond angles of ethene. C2H4 Molecular Geometry And Bond Angles.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
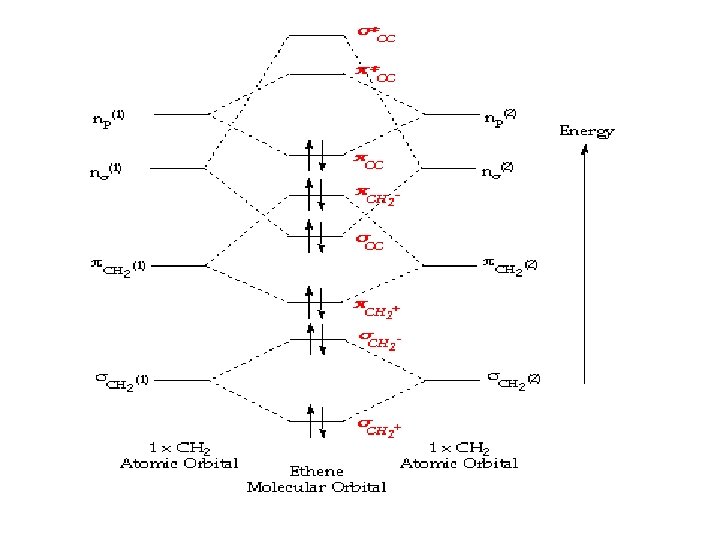
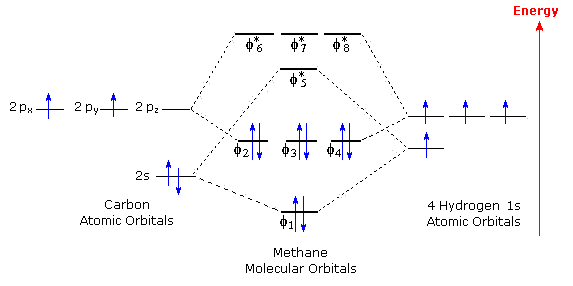
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Ethylene E E H2CCH2 p 2 sp2 1s p 2 sp2 1s σ σ π π AO. Ethylene Ethene Phophorus Pentafluoride. For the ethene orbital energy diagram these are shown as p CC for the HOMO and p CC for the LUMO. Consider the pi bond of ethene in simple molecular orbital terms The qualitative results would be the same for any pi or sigma bond.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Make a list of all the molecular orbitals in the molecule. A Molecular Approach p. There is a formation of a sigma bond and a pi bond between two carbon atoms. Ethene is built from hydrogen atoms 1s 1 and carbon atoms 1s 2 2s 2 2p x 1 2p y 1. ψ1 and ψ2 also referred to as π1 and π2.
 Source: researchgate.net
Source: researchgate.net
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. σ CH3 and π CH3 orbitals are primarily C-H bonding - do not. Arrange the molecular orbitals in order using the general order on the previous page. Means molecular orbitals σ π C from left end of double bond MO. A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals.
 Source: friendlychemistry.blogspot.com
Source: friendlychemistry.blogspot.com
ψ1 and ψ2 also referred to as π1 and π2. ZFor ethene the σιγµα framework is created by the interaction of the sp2 hybrid orbitals of the C atoms and H1s orbitals. This will be done by combining two methylene. σ CH3 and π CH3 orbitals are primarily C-H bonding - do not. Pi Molecular Orbitals of Ethylene.
 Source: chegg.com
Source: chegg.com
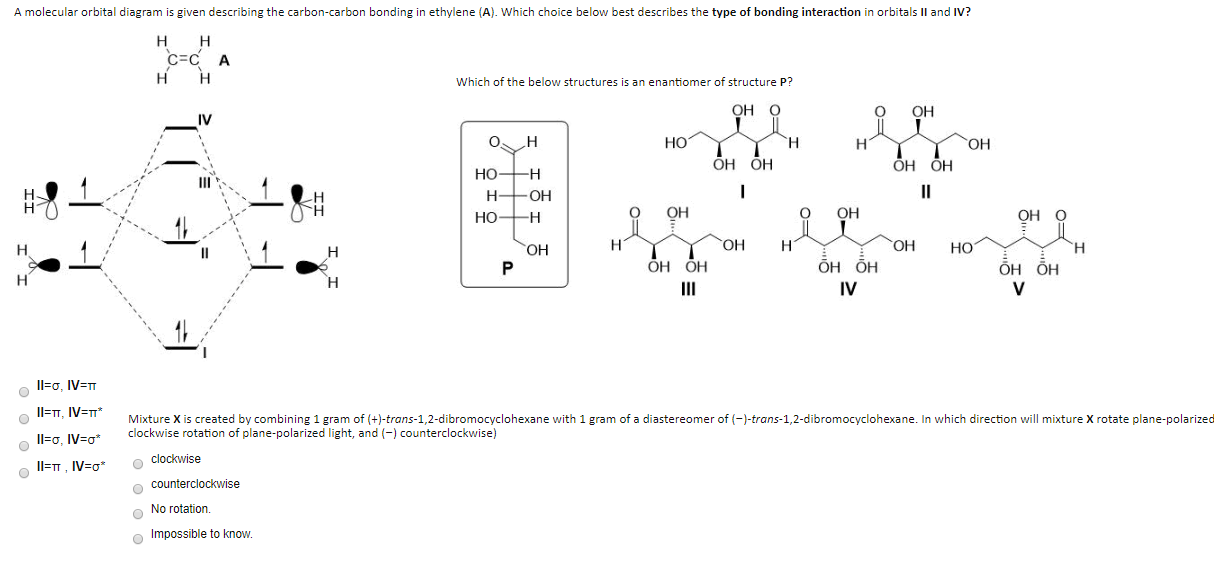
Pi Molecular Orbitals of Ethylene. The out-of-phase combination the anti-bonding orbital. If playback doesnt begin shortly try restarting your device. McQuarrie Simon Physical Chemistry. In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals.
 Source: tutorsglobe.com
Source: tutorsglobe.com
The above diagram shows the Molecular OrbitalMO diagram of etheneethylene. The in-phase combination gave the bonding orbital. Count the orbitals to make sure that you havent forgotten any. The out-of-phase combination the anti-bonding orbital. How many molecular orbitals must there be Steps 34.
 Source: studyorgo.com
Source: studyorgo.com
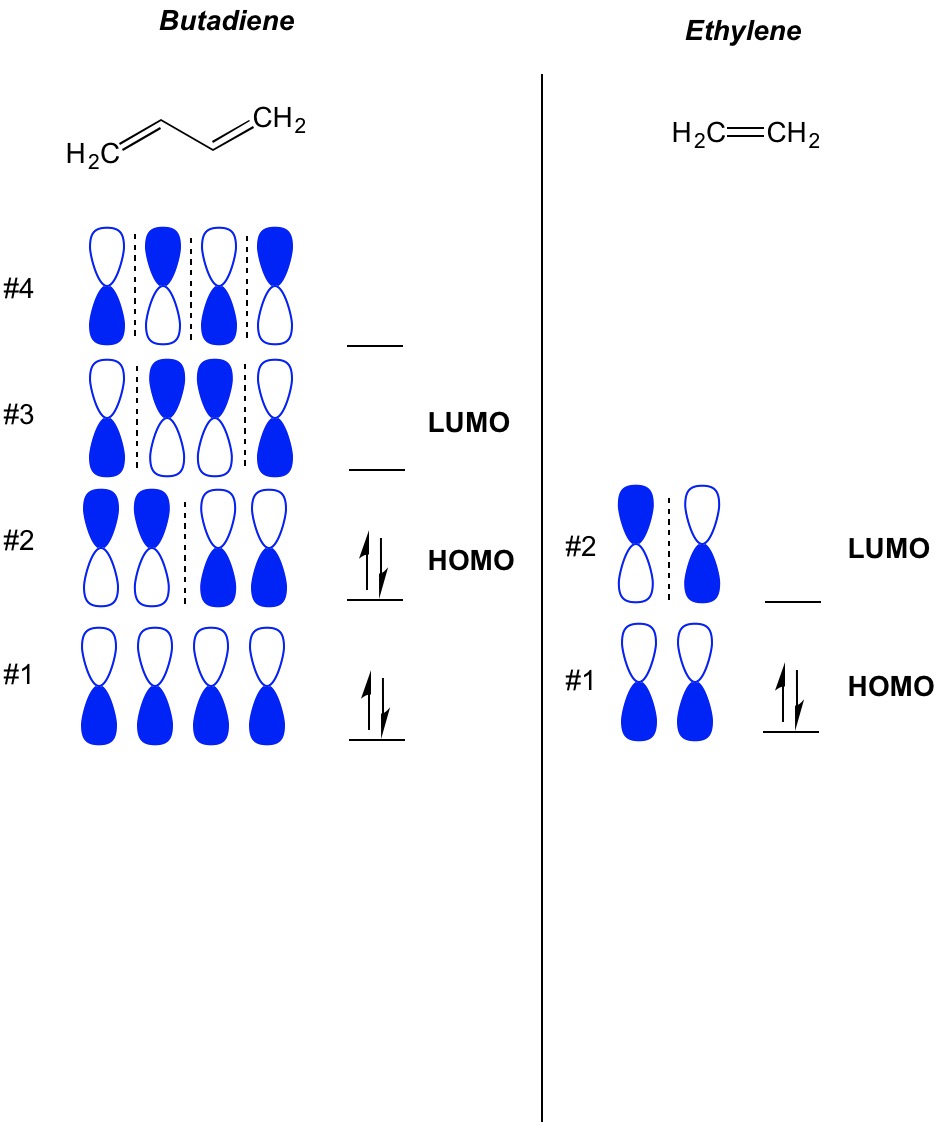
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. How many molecular orbitals must there be Steps 34. Jmol_Canvas2D Jmol Ethylene x. The diagram below shows the bond lengths and hydrogen-carbon-carbon bond angles of ethene. Its chemistry is dominated by two frontier orbitals that is the Highest Occupied Molecular Orbital HOMO and the Lowest Unoccupied Molecular Orbital LUMO.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
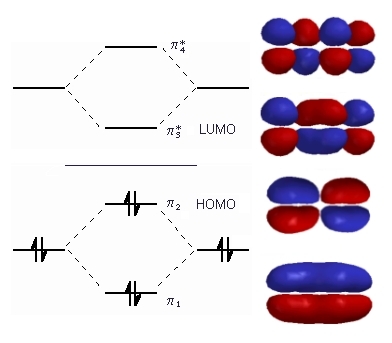
Q The overlap of the two atomic orbitals AOs results in the formation of two molecular orbitals MOs one of which is lower in energy than the original AOs the bonding MO or BMO and the other higher. σ CH3 and π CH3 orbitals are primarily C-H bonding - do not. Fill the molecular orbitals with the correct number of electrons. σ out is directed away from hydrogens and towards the C-C bond. π Molecular Orbitals of Ethene.
 Source: slidetodoc.com
Source: slidetodoc.com
McQuarrie Simon Physical Chemistry. Count the orbitals to make sure that you havent forgotten any. Pi Molecular Orbitals of Ethylene. C C HHC C C from other end of double bond AO. There is a formation of a sigma bond and a pi bond between two carbon atoms.
 Source: researchgate.net
Source: researchgate.net
Consider the pi bond of ethene in simple molecular orbital terms The qualitative results would be the same for any pi or sigma bond. In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals. Fill the molecular orbitals with the correct number of electrons. At this stage we note that from our N pz orbitals we will obtain N π orbitals. C2H4 Molecular Geometry And Bond Angles.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
In ethene molecule the carbon atoms are sp 2 hybridized. Looking at both sigma and pi bonds. Ethene is built from hydrogen atoms 1s 1 and carbon atoms 1s 2 2s 2 2p x 1 2p y 1. Hückel theory for ethylene we find that a single ethylene double bond has an energy ECC 2α 2β Thus if benzene simply had three double bonds we would expect. There is a formation of a sigma bond and a pi bond between two carbon atoms.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
C C HHC C C from other end of double bond AO. This will be done by combining two methylene. Ethylene E E H2CCH2 p 2 sp2 1s p 2 sp2 1s σ σ π π AO. Make a list of all the molecular orbitals in the molecule. Ethene is built from hydrogen atoms 1s 1 and carbon atoms 1s 2 2s 2 2p x 1 2p y 1.
 Source: chegg.com
Source: chegg.com
At this stage we note that from our N pz orbitals we will obtain N π orbitals. A Molecular Approach p. Looking at both sigma and pi bonds. Introduction to Molecular Orbital Theory. A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals.
 Source: youtube.com
Source: youtube.com
Ethene Ethylene Ethylene MOs. In this manner ethane can be constructed from MOs of two. ZFor ethene the σιγµα framework is created by the interaction of the sp2 hybrid orbitals of the C atoms and H1s orbitals. Fill the molecular orbitals with the correct number of electrons. HÜCKEL MOLECULAR ORBITAL THEORY.
 Source: researchgate.net
Source: researchgate.net
In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals. Looking at both sigma and pi bonds. σ CH3 and π CH3 orbitals are primarily C-H bonding - do not. Count the orbitals to make sure that you havent forgotten any. At this stage we note that from our N pz orbitals we will obtain N π orbitals.

How many molecular orbitals must there be Steps 34. Count the orbitals to make sure that you havent forgotten any. Polarity of C2H4 The C2H4 molecule is non-polar in nature as all the atoms are symmetrically arranged across the molecule and both carbon atoms have the same influence on the bonded electrons. How many molecular orbitals must there be Steps 34. In this video we will generate a qualitative MO diagram of ethene through a fragment molecular orbital approach.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ethylene molecular orbital diagram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.