Free energy reaction coordinate diagram
Home » Wallpapers » Free energy reaction coordinate diagramYour Free energy reaction coordinate diagram images are ready in this website. Free energy reaction coordinate diagram are a topic that is being searched for and liked by netizens today. You can Download the Free energy reaction coordinate diagram files here. Download all royalty-free vectors.
If you’re searching for free energy reaction coordinate diagram images information connected with to the free energy reaction coordinate diagram interest, you have come to the right site. Our site always gives you suggestions for viewing the highest quality video and picture content, please kindly hunt and find more enlightening video articles and images that match your interests.
Free Energy Reaction Coordinate Diagram. Below is a reaction coordinate diagram for an endothermic reaction. Which of the following is an energy diagram for a two-step reaction. It is usually a geometric parameter that changes during the. It shows how the energy of the system changes during a chemical reaction.
 31 Label The Following Reaction Coordinate Diagram Label Design Ideas 2020 From dandelionsandthings.blogspot.com
31 Label The Following Reaction Coordinate Diagram Label Design Ideas 2020 From dandelionsandthings.blogspot.com
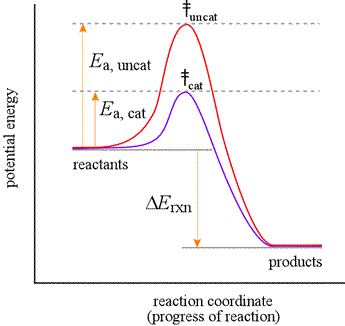
Energy profile chemistry From Wikipedia the free encyclopedia. A measure of freedom of motion ΔGo ΔHo - TΔSo ΔGΔHΔS ΔE are state functions. ΔG cat ΔG uncat. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism. Diagram of a catalytic reaction showing the energy niveau depending on the reaction coordinate. Energy diagrams for these processes will often plot the enthalpy H instead of Free Energy for simplicityThe standard Gibbs Free Energy change for a reaction can be related to the reactions equilibrium constant K eq by a simple equationΔG -RT ln K eq where.
Free Energy G Reaction Coordinate D TS in N Fig.
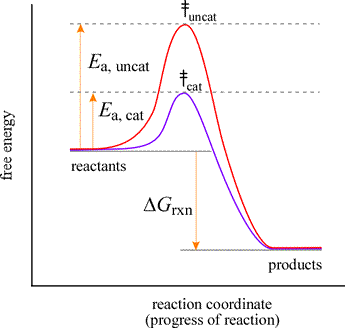
Figure 8-3 Reaction coordinate diagram for a chemical reaction. Plot A represents a secondary alkyl halide starting material and plot B a tertiary alkyl halide starting material. The free energy of the system is plotted agains the progress of the reaction. It is usually a geometric parameter that changes during the. For a catalysed reaction the activation energy is lower. ΔG cat ΔG uncat.
 Source: kindpng.com
Source: kindpng.com
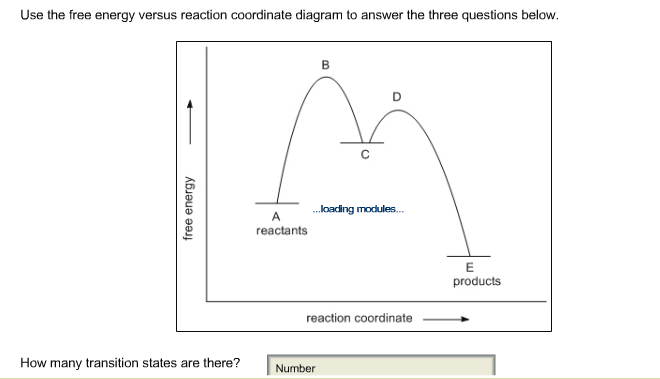
A reaction coordinate diagram taking starting material S to product P. Energy diagrams for these processes will often plot the enthalpy H instead of Free Energy for simplicityThe standard Gibbs Free Energy change for a reaction can be related to the reactions equilibrium constant K eq by a simple equationΔG -RT ln K eq where. A measure of freedom of motion ΔGo ΔHo - TΔSo ΔGΔHΔS ΔE are state functions. Reaction coordinate diagram for an enzyme-catalyzed reaction representing the mechanistic model we are considering. The equilibrium constant for the reaction is determined by G and the free energy of activation is G.
 Source: varsitytutors.com
Source: varsitytutors.com
Free Energy Reaction Coordinate G G S P X Figure K1. Select all that apply. Home Free Energy Reaction Coordinate Diagram Endergonic Free Energy Reaction Coordinate Diagram Endergonic Written By JupiterZ Thursday December 20. The diagram could represent the addition of HCl to propene. Gibbs free energy reaction coordinate profiles found in some textbooks.
 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
A reaction coordinate diagram taking starting material S to product P. Free Energy G Reaction Coordinate D TS in N Fig. So the first step in the reaction corden up diagram shows here that the greatest free energy of activation is in the forward direction. Select all that apply. The diagram could represent different S_N 1 reactions.
 Source: studyorgo.com
Source: studyorgo.com
Free energy diagram to illustrate the higher unfolding energy barrier for a kinetically stable protein under native conditions as compared to that of a normal protein represented by the dash line. A measure of freedom of motion ΔGo ΔHo - TΔSo ΔGΔHΔS ΔE are state functions. Although the equations look similar it is. Figure 8-3 Reaction coordinate diagram for a chemical reaction. This is an exothermic reactionheat is given off and should be.
 Source: varsitytutors.com
Source: varsitytutors.com
Energy diagrams for these processes will often plot the enthalpy H instead of Free Energy for simplicityThe standard Gibbs Free Energy change for a reaction can be related to the reactions equilibrium constant K eq by a simple equationΔG -RT ln K eq where. Draw a reaction coordinate diagram for this reaction as above but add the activation energy e a for the catalyzed reaction on the appropriate curve in this diagram and label it. A measure of freedom of motion ΔGo ΔHo - TΔSo ΔGΔHΔS ΔE are state functions. It shows how the energy of the system changes during a chemical reaction. Equation 1 is an empirical one that does not consider entropy.
 Source: differencebetween.com
Source: differencebetween.com
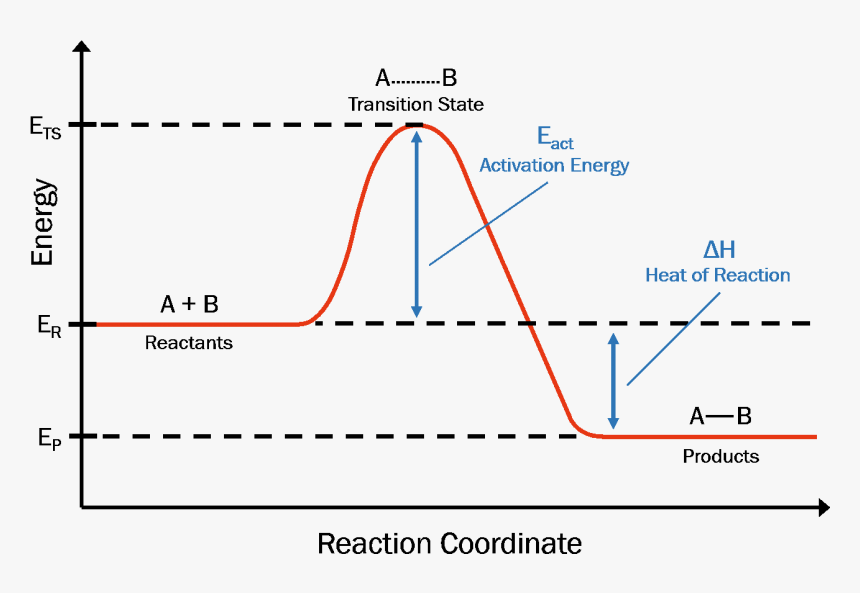
Ron Rusay A Reaction Coordinate Energy Diagram Thermodynamic Quantities Gibbs standard free energy change ΔGo Enthalphy ΔHo. Reaction coordinate diagram for an enzyme-catalyzed reaction representing the mechanistic model we are considering. The diagram below is called a reaction coordinate diagram. The fully filled in reaction coordinate diagram is displayed below. A diagram of this kind is a description of the energetic course of the reaction and the horizontal axis reaction coordinate reflects the progressive chemical changes eg bond breakage or formation.
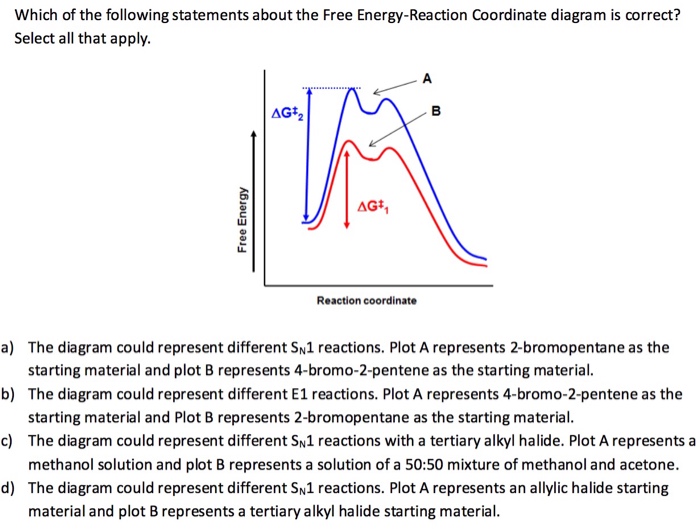
The fast formed intermediate B is found to be more apt to revert to react INTs or go on to form products as the energy barrier from this transition state is lasted on the energy Bari from the second transition state. The equilibrium constant for the reaction is determined by G and the free energy of activation is G. It is usually a geometric parameter that changes during the. Which of the following statements about the Free Energy-Reaction Coordinate diagram is correct. Figure 8-3 Reaction coordinate diagram for a chemical reaction.
 Source: researchgate.net
Source: researchgate.net
Diagram of a catalytic reaction showing the energy niveau depending on the reaction coordinate. A diagram of this kind is a description of the energetic course of the reaction and the horizontal axis reaction coordinate reflects the progressive chemical changes eg bond breakage or formation. A typical reaction coordinate diagram for a mechanism with a single step is shown below. A reaction coordinate diagram taking starting material S to product P. Energy profile chemistry From Wikipedia the free encyclopedia.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Which of the following statements about the Free Energy-Reaction Coordinate diagram is correct. Free energy diagram to illustrate the higher unfolding energy barrier for a kinetically stable protein under native conditions as compared to that of a normal protein represented by the dash line. Which of the following is an energy diagram for a two-step reaction. The transition state is the highest energy structure on the reaction coordinate and is labeled. The diagram could represent different S_N 1 reactions.
 Source: chegg.com
Source: chegg.com
Free energy diagram to illustrate the higher unfolding energy barrier for a kinetically stable protein under native conditions as compared to that of a normal protein represented by the dash line. Below is a reaction coordinate diagram for an endothermic reaction. Free Energy G Reaction Coordinate D TS in N Fig. Which of the following statements about the Free Energy-Reaction Coordinate diagram is correct. In chemistry a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway.
 Source: clutchprep.com
Source: clutchprep.com
The diagram could represent the addition of HCl to propene. A measure of freedom of motion ΔGo ΔHo - TΔSo ΔGΔHΔS ΔE are state functions. The heat given off or absorbed during a reaction Entropy ΔSo. The energy diagram for a reaction model consisting of one enzyme one substrate and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. Plot A represents a secondary alkyl halide starting material and plot B a tertiary alkyl halide starting material.
 Source: wikiwand.com
Source: wikiwand.com
It shows how the energy of the system changes during a chemical reaction. Draw a reaction coordinate diagram for this reaction as above but add the activation energy e a for the catalyzed reaction on the appropriate curve in this diagram and label it. The energy diagram for a reaction model consisting of one enzyme one substrate and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. In this example B is at a lower total energy than A. The vertical axis represents the overall energy of the reactants while the horizontal axis is thereaction coordinate indicating that the change in standard Gibbs Free Energy for the reaction u0394Gu02darnx is negative in an energy diagram.
 Source: wikiwand.com
Source: wikiwand.com
The transition state is the highest energy structure on the reaction coordinate and is labeled. It is usually a geometric parameter that changes during the. Which of the following statements about the Free Energy-Reaction Coordinate diagram is correct. The free energy of the system is plotted agains the progress of the reaction. The equilibrium constant for the reaction is determined by G and the free energy of activation is G.
 Source: youtube.com
Source: youtube.com
Free energy kJmol reaction coordinate A. The free energy of activation ΔG which for our present purposes can be considered as equivalent to activation energy is much lower for the catalyzed reaction compared with the uncatalyzed reaction. This is an exothermic reactionheat is given off and should be. Plot A represents a secondary alkyl halide starting material and plot B a tertiary alkyl halide starting material. A typical reaction coordinate diagram for a mechanism with a single step is shown below.
 Source: chemgapedia.de
Source: chemgapedia.de
The diagram could represent different S_N 1 reactions. If you substitute it for the Eyring equation considering free energy of activation Delta Gddagger you end up with equation 6 as expected not equation 5Wikipedia has a nice section on the relationship of activation energy and Gibbs energy of activation. In this lesson we will learn about reaction coordinate diagrams and how they An endothermic reaction is one where at the end of the reaction energy is put. Free energy diagram to illustrate the higher unfolding energy barrier for a kinetically stable protein under native conditions as compared to that of a normal protein represented by the dash line. Draw a reaction coordinate diagram for this reaction as above but add the activation energy e a for the catalyzed reaction on the appropriate curve in this diagram and label it.
 Source: organicchemistrytutor.com
Source: organicchemistrytutor.com
The free energy of activation ΔG which for our present purposes can be considered as equivalent to activation energy is much lower for the catalyzed reaction compared with the uncatalyzed reaction. In this example B is at a lower total energy than A. A typical reaction coordinate diagram for a mechanism with a single step is shown below. The diagram could represent the addition of HCl to propene. Free Energy G Reaction Coordinate D TS in N Fig.
 Source: wikiwand.com
Source: wikiwand.com
In this example B is at a lower total energy than A. For a catalysed reaction the activation energy is lower. The energy diagram for a reaction model consisting of one enzyme one substrate and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. It is usually a geometric parameter that changes during the. Select all that apply.
 Source: guweb2.gonzaga.edu
Source: guweb2.gonzaga.edu
K eq product reactant at equilibrium. Home Free Energy Reaction Coordinate Diagram Endergonic Free Energy Reaction Coordinate Diagram Endergonic Written By JupiterZ Thursday December 20. A diagram of this kind is a description of the energetic course of the reaction and the horizontal axis reaction coordinate reflects the progressive chemical changes eg bond breakage or formation. Select all that apply. The diagram could represent the addition of HCl to propene.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title free energy reaction coordinate diagram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.